- Product Listings
- Compare Products
- Calculators
- Product Tours
- Expert Reviews
- Our Experts
- Company Directory
- About Beye
- Contact Us
Ⓒ 2026 Beye.com. All rights reserved.
This content is intended for health care professionals and providers only. The information contained on Beye.com, including text, graphics, images, and interactive activities, is for informational purposes only, and is not intended to be a substitute for professional medical advice. Beye LLC, via its Editors and Publisher, accepts no responsibility for any injury or damage to persons or property occasioned through the implementation of any ideas or use of any product described herein. Although great care is taken to ensure that all information is accurate, it is recommended that readers seek independent verification of advice on drugs and other product usage, surgical techniques and clinical processes prior to their use. References made in article may indicate usage of medical equipment or drugs at dosages, for periods of time, and in combination not included in the current prescribing information. Inclusion of advertising materials on the website thereof, does not constitute and representation or guarantee by Beye LLC of the quality of such products, or of the claims made.
Compare Pharmaceuticals
4 Products
reset all
At-a-Glance
Description
FDA
CE Mark
Active Ingredients
Application
Status
Strength
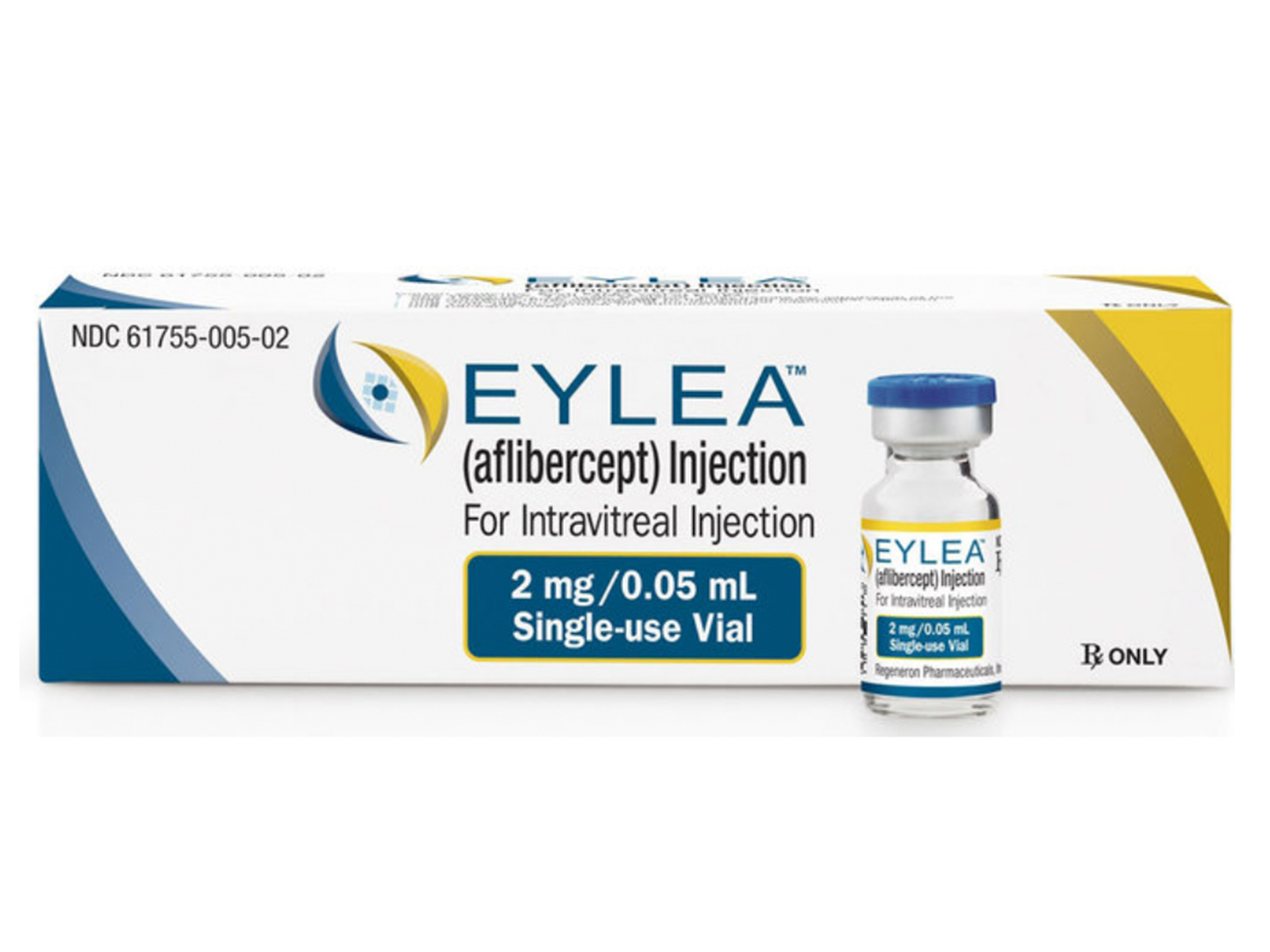
- Mechanism of Action: Vascular endothelial growth factor-A (VEGF-A) and placental growth factor (PlGF) are members of the VEGF family of angiogenic factors that can act as mitogenic, chemotactic, and vascular permeability factors for endothelial cells. VEGF acts via two receptor tyrosine kinases, VEGFR-1 and VEGFR-2, present on the surface of endothelial cells. PlGF binds only to VEGFR-1, which is also present on the surface of leucocytes. Activation of these receptors by VEGF-A can result in neovascularization and vascular permeability. Aflibercept acts as a soluble decoy receptor that binds VEGF-A and PlGF, and thereby can inhibit the binding and activation of these cognate VEGF receptors.
- Dosage:
- Neovascular (Wet) Age-Related Macular Degeneration (AMD): 2 mg (0.05 mL) administered by intravitreal injection every 4 weeks (monthly) for the first 3 months, followed by 2 mg (0.05 mL) via intravitreal injection once every 8 weeks (2 months).
- Macular Edema Following Retinal Vein Occlusion (RVO): 2 mg (0.05 mL) administered by intravitreal injection once every 4 weeks (monthly).
- Diabetic Macular Edema (DME) and Diabetic Retinopathy (DR) in Patients with Diabetic Macular Edema: 2 mg (0.05 mL) administered by intravitreal injection every 4 weeks (monthly) for the first 5 injections followed by 2 mg (0.05 mL) via intravitreal injection once every 8 weeks (2 months).
Eylea (aflibercept) Injection is indicated for the treatment of neovascular (Wet) age-related macular degeneration (AMD), macular edema following retinal cein occlusion (RVO), diabetic macular edema (DME), and diabetic retinopathy (DR) in patients with DME.
Yes
Not specified
Aflibercept
Intravitreal
Prescription
Not specified
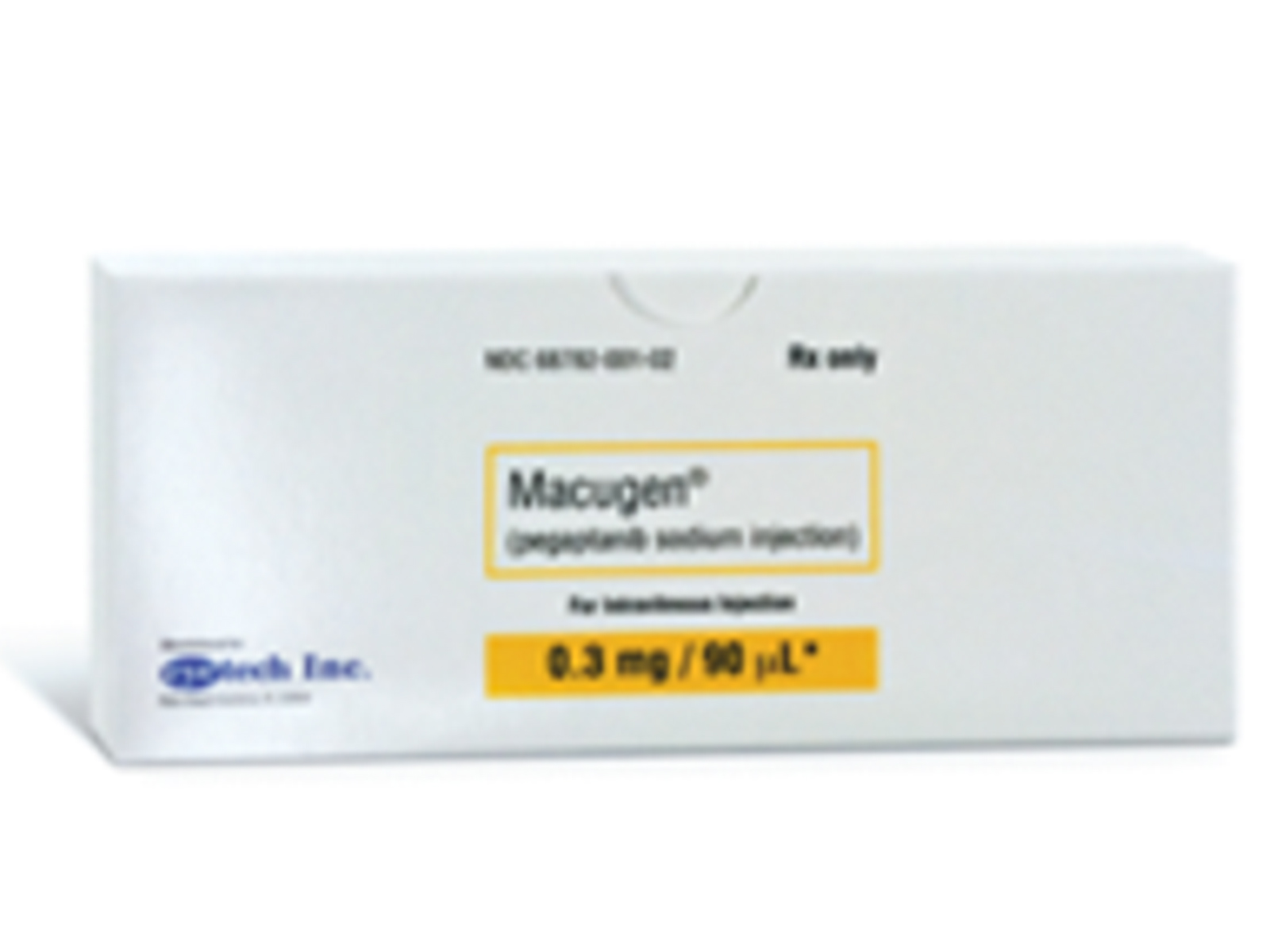
- Mechanism of Action: Pegaptanib is a selective vascular endothelial growth factor (VEGF) antagonist. It is an aptamer, a pegylated modified oligonucleotide, which adopts a three-dimensional conformation that enables it to bind to extracellular VEGF.
- Dosage
Macugen (pegaptanib sodium injection) is indicated for the treatment of neovascular (wet) age-related macular degeneration.
Yes
Yes
Pegaptanib sodium
Intravitreal
Prescription
EQ 0.3MG ACID/0.09ML
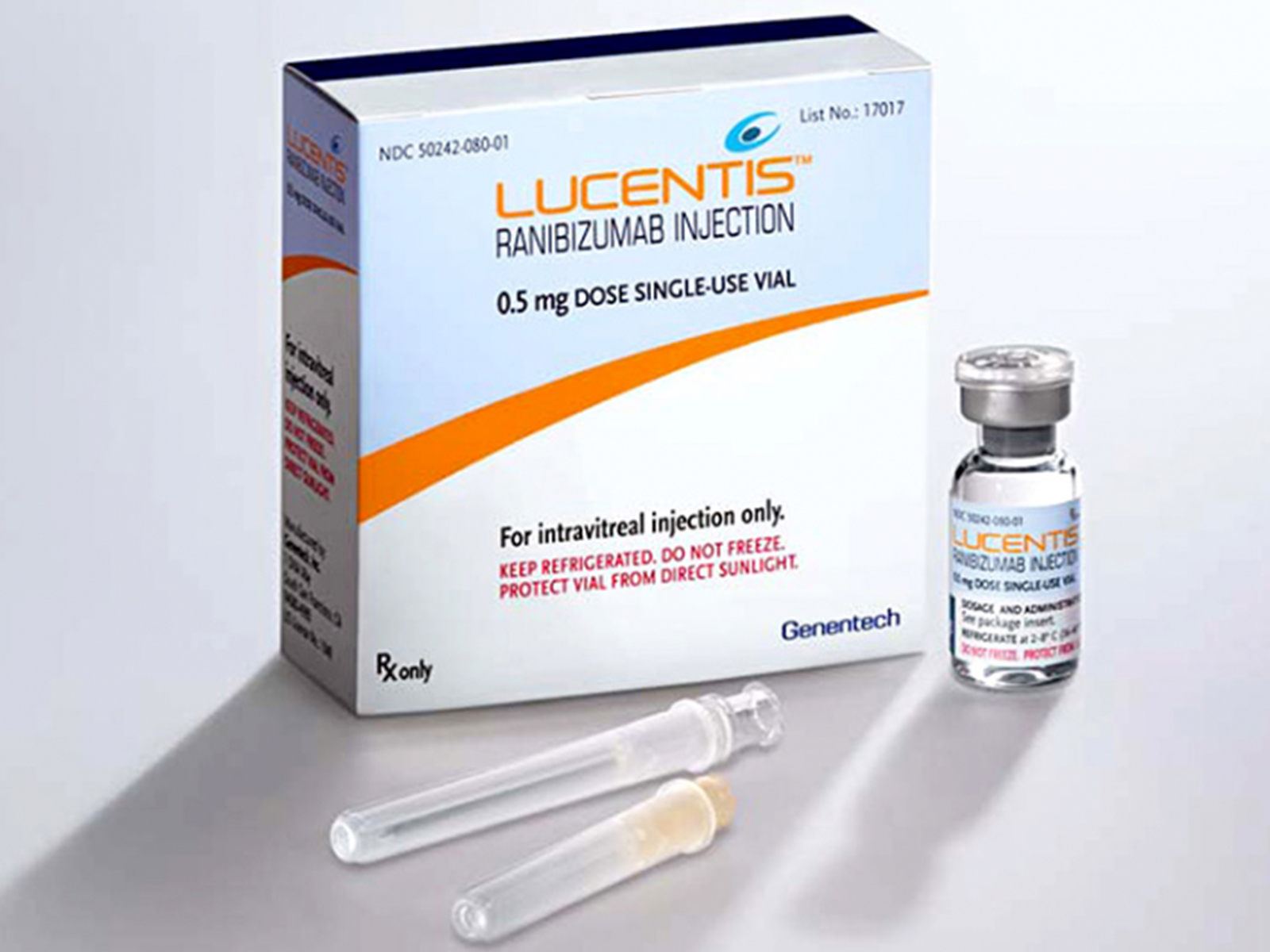
- Mechanism of Action: Ranibizumab binds to the receptor binding site of active forms of VEGF-A, including the biologically active, cleaved form of this molecule, VEGF. The binding of ranibizumab to VEGF-A prevents the interaction of VEGF-A with its receptors (VEGFR1 and VEGFR2) on the surface of endothelial cells, reducing endothelial cell proliferation, vascular leakage, and new blood vessel formation.
- Dosage:
- Neovascular (Wet) Age-Related Macular Degeneration (AMD): 0.5 mg (0.05 mL) to be administered by intravitreal injection once a month (approximately 28 days).
- Macular Edema Following Retinal Vein Occlusion (RVO): 0.5 mg (0.05 mL) to be administered by intravitreal injection once a month (approximately 28 days).
- Diabetic Macular Edema (DME) and Diabetic Retinopathy (DR): 0.3 mg (0.05 mL) to be administered by intravitreal injection once a month (approximately 28 days).
- Myopic Choroidal Neovascularization (mCNV): 0.5 mg (0.05 mL) to be initially administered by intravitreal injection once a month (approximately 28 days) for up to three months. Patients may be retreated if needed.
Lucentis (ranibizumab injection) is a a vascular endothelial growth factor (VEGF) inhibitor indicated for the treatment of patients with neovascular (wet) age-related macular degeneration (AMD), macular edema following retinal vein occlusion (RVO), diabetic macular edema (DME), diabetic retinopathy (DR), and myopic choroidal neovascularization (mCNV).
Yes
Not specified
Ranibizumab
Intravitreal
Prescription
Not specified
Mechanism of Action: Bevacizumab binds VEGF and prevents the interaction of VEGF to its receptors (Flt-1 and KDR) on the surface of endothelial cells. The interaction of VEGF with its receptors leads to endothelial cell proliferation and new blood vessel formation in in vitro models of angiogenesis.
Avastin (bevacizumab) injection is a vascular endothelial growth factor directed antibody indicated for the treatment of metastatic colorectal cancer and metastatic colorectal cancer and used off-label to treat age-related macular degeneration (AMD).
Yes (not for ophthalmic use)
Not specified
Bevacizumab
Intravitreal
Prescription
Not specified